Pyrolysis, a process as old as the discovery of fire itself, involves the thermal decomposition of organic materials in the absence of oxygen. This fascinating chemical transformation holds significant implications for various industries, from biofuel production to waste management. In this article, we delve into the chemistry behind pyrolysis, unraveling the intricate reactions that occur as organic matter undergoes thermal degradation.
Understanding Pyrolysis
At its core, pyrolysis is a complex series of chemical reactions driven by heat. When organic materials such as biomass, plastics, or rubber are subjected to high temperatures in the absence of oxygen, they undergo thermal decomposition in pyrolysis plant, yielding a mixture of gases, liquids, and solids.
Types of Pyrolysis
Pyrolysis can be categorized into three main types based on the operating conditions: slow, fast, and flash pyrolysis. Slow pyrolysis involves heating biomass at relatively low temperatures (300-500°C) over an extended period, resulting in the production of carbon black, pyrolysis oil, and syngas. Fast pyrolysis occurs at higher temperatures (500-800°C) and shorter residence times, leading to the predominant formation of pyrolysis oil. Flash pyrolysis operates at even higher temperatures (>800°C) and extremely short residence times, favoring the production of gases such as hydrogen and methane.
Chemical Reactions in Pyrolysis
The chemical reactions that take place during pyrolysis are highly complex and involve a multitude of pathways. However, several key reactions dominate the process, leading to the formation of valuable products.
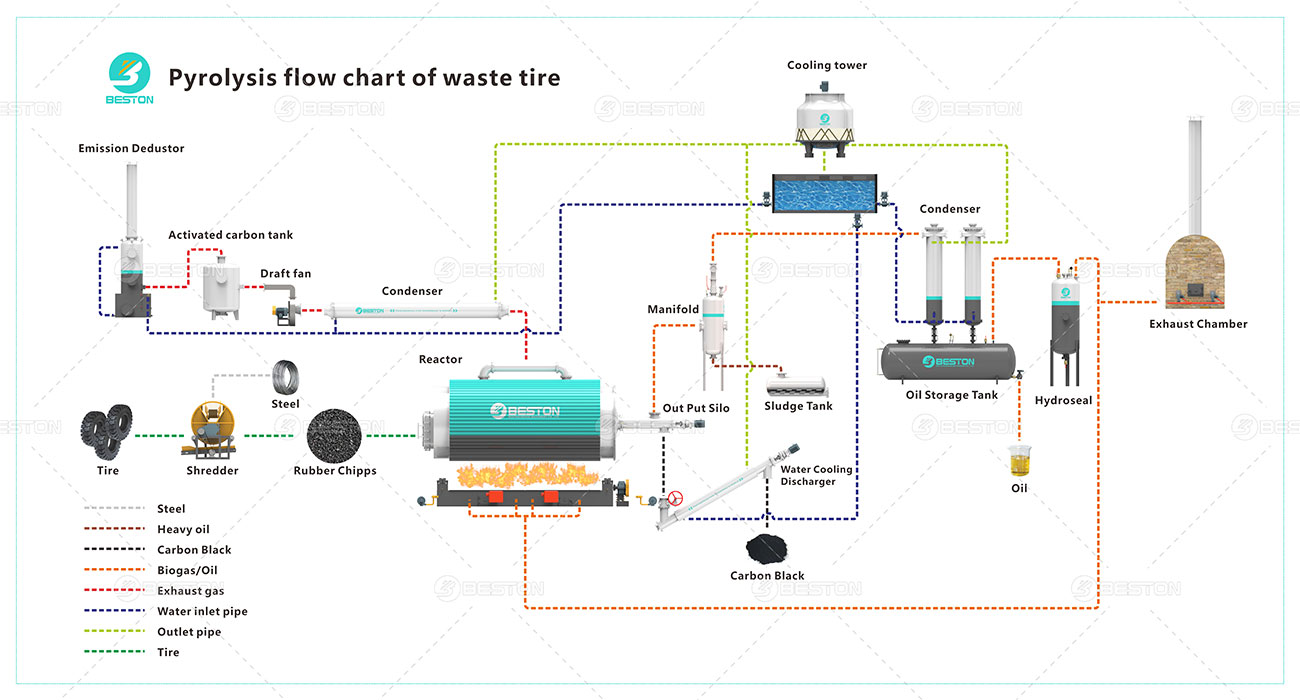
Dehydration and Decarboxylation
During pyrolysis, organic materials undergo dehydration and decarboxylation reactions. Dehydration involves the removal of water molecules from the biomass, resulting in the formation of volatile compounds such as alcohols, ketones, and aldehydes. Decarboxylation, on the other hand, entails the elimination of carboxyl groups (-COOH) from organic acids, yielding carbon dioxide and various volatile organic compounds.
Cracking and Polymerization
As the temperature increases, larger organic molecules undergo cracking reactions, breaking down into smaller, more volatile compounds. This process results in the formation of gases such as carbon monoxide, hydrogen, methane, and ethylene. Conversely, some compounds may undergo polymerization, wherein smaller molecules combine to form larger, more complex structures such as char and pyrolysis oil.
Products of Pyrolysis
The products obtained from plastic/tyre/oil sludge pyrolysis plant vary depending on the type of feedstock and operating conditions. However, they generally consist of three main fractions: carbon black, pyrolysis oil, and syngas.
Carbon black
Carbon black is a carbon-rich solid residue produced during pyrolysis. It is characterized by its high surface area, porosity, and stability, making it an ideal soil amendment for improving soil fertility and carbon sequestration.
Pyrolysis oil
Pyrolysis oil, also known as pyrolysis oil or biocrude, is a dark, viscous liquid obtained from the condensation of vapors generated during pyrolysis. It contains a complex mixture of oxygenated hydrocarbons, including aldehydes, ketones, phenols, and acids, making it a potential feedstock for biofuel production.
Syngas
Syngas, or synthesis gas, is a mixture of carbon monoxide, hydrogen, and traces of methane produced during pyrolysis. It can be used as a fuel for heat and power generation or further processed into chemicals and fuels through processes such as Fischer-Tropsch synthesis.

Applications of Pyrolysis
Tyre/oil sludge/plastic to oil machine finds applications across a wide range of industries, offering solutions to environmental challenges and resource utilization.
Biofuel Production
Carbon black, pyrolysis oil, and syngas obtained from pyrolysis can serve as feedstocks for biofuel production, offering a renewable alternative to fossil fuels.
Waste Management
Pyrolysis provides a sustainable solution to the management of organic waste streams, converting biomass, plastics, and rubber into valuable products while reducing landfill waste and greenhouse gas emissions.
Soil Amendment
Carbon black produced through pyrolysis can improve soil fertility, structure, and carbon sequestration capacity, making it a valuable soil amendment for agricultural and environmental applications. For the latest updates and detailed information, be sure to visit Beston Group.